There are two kinds of electric charge: positive and negative. Particles with like charges will repel each other, and particles with unlike charges attract each other. We generally approximate charges as 'point particles', or objects with radii and masses so small in comparison to other distances and masses used in problems that they are inconsequential. The object is then treated as if all its charge and mass were concentrated at a single point. In many of the problems we will encounter, protons and electrons are treated as point charges.

Two charges will exert a force on each other that depends on the sign of the charges. This force will be attractive when the two charges are opposite, and repulsive when the two charges are the same. The strength of this electrical interaction is a vector quantity that has magnitude and direction. Each individual charge will additionally create an Electric Field around it, whose magnitude depends on the sign and magnitude of the charge, and which decreases in magnitude radially from the source charge. If the electric field at a particular location is known, then this field can be used to calculate the electric force of the particle being acted upon. The electric force is directly proportional to the amount of charge within each particle being acted upon by the other's electric field. Moreover, the magnitude of the force is inversely proportional to the square distance between the two interacting particles. It is important to remember that a particle cannot have an electric force on itself; there must be at least two interacting, charged components.
The Coulomb Force Law
The formula for the magnitude of the electric force between two point charges is:
Where [math]\displaystyle< _ >[/math] and [math]\displaystyle< _ >[/math] are the magnitudes of electric charge of point 1 and point 2, and [math]\displaystyle< r >[/math] is the distance between the two point charges. The units for electric force are the same as the units for all forces: Newtons. The expression [math]\displaystyle< \frac >[/math] is known as the electric constant and carries the value 9e9. Epsilon naught defines the electric permittivity of free space (the permittivity of air - this constant has different values if the force is acting in different materials!).
Interestingly enough, one can see a relationship between this formula and the formula for gravitational force ( [math]\displaystyle < F=\frac<|__|> >[/math] ). From this relationship, one can conclude that the interactions of two objects as a result of their charges or masses follow similar fundamental laws of physics. Namely, these two forces are both examples of the inverse square law.
Derivations of Electric Force
The electric force on a particle can also be written as:
Where [math]\displaystyle< q >[/math] is the charge of the particle and [math]\displaystyle< \vec E >[/math] is the external electric field. It is interesting to note that the electric field from a charge( [math]\displaystyle < q_>[/math] ) takes the form shown below:
Therefore, the magnitude of electric force between point charge 1 and point charge 2 can be written as:
The units of charge are in Coulombs and the units for electric field are in Newton/Coulombs, so this derivation is correct in its dimensions since multiplying the two units gives just Newtons.
A computational representation of the Electric force can be created using VPython. The code below shows how we can find the net force, momentum and final position between two charged particles in VPython. This example uses two positively charged protons.
#CONSTANTS E= 9e9 # Electric Force constant q1 = +1.6e-19 # Charge of proton 1 q2 = +1.6e-19 # Charge of proton 2
while t < 200 : # Calculate electric force acting on proton 1 by proton 2 r = proton.pos - proton2.pos rmag = mag(r) rhat = r/rmag Fnet = E*((q1*q2)/(mag2(r)))*rhat # Calculate electric force acting on proton 1 by proton 2 r2 = proton2.pos - proton.pos rmag2 = mag(r2) rhat2 = r2/rmag2 Fnet2 = E*((q1*q2)/(mag2(r2)))*rhat2 # Update positions of BOTH protons pproton = pproton + Fnet*deltat vproton = pproton/mproton proton.pos = proton.pos + vproton*deltat pproton2 = pproton2 + Fnet2*deltat vproton2 = pproton2/mproton proton2.pos = proton2.pos + vproton2*deltat
Notice that this iterative process is very similar to that working with Gravitational Forces.
Notice, however, that the direction of motion of each particle is not determined by relative position, but by the charges of the particles. The product of these charges ultimately determine whether the two particles will attract each other, or repel. This is demonstrated in the following simulations:
The trinket model linked demonstrates the Electric force interaction of a proton and an electron. Proton-Electron Electric Force Interaction
The trinket model linked demonstrates the Electric force interaction between two protons. Proton-Proton Electric Force Interaction
Problem: Find the electric force of a -3 C particle in a region with an electric field of [math]\displaystyle< \lt 7, 5, 0\gt >[/math] N/C.
Step 1: Substitute values into the correct formula.
The electric force vector for this particle is [math]\displaystyle< \lt -21, -15, 0\gt >[/math] N.
Problem: Find the magnitude of electric force on two charged particles located at [math]\displaystyle< \lt 0, 0, 0\gt >[/math] m and [math]\displaystyle< \lt 0, 10, 0\gt >[/math] m. The first particle has a charge of +5 nC and the second particle has a charge of -10 nC. Is the force attractive or repulsive?
Step 1: Find the distance between the two point charges.
The distance between the two points is 10 m.
Step 2: Substitute values into the correct formula.
The magnitude of electric force is [math]\displaystyle< |\vec F|=4.5e-9 >[/math] N.
Step 3: Determine if force is attractive or repulsive.
Since the first particle is positively charged and the second is negatively charged, the force is attractive. The particles are attracted to each other.
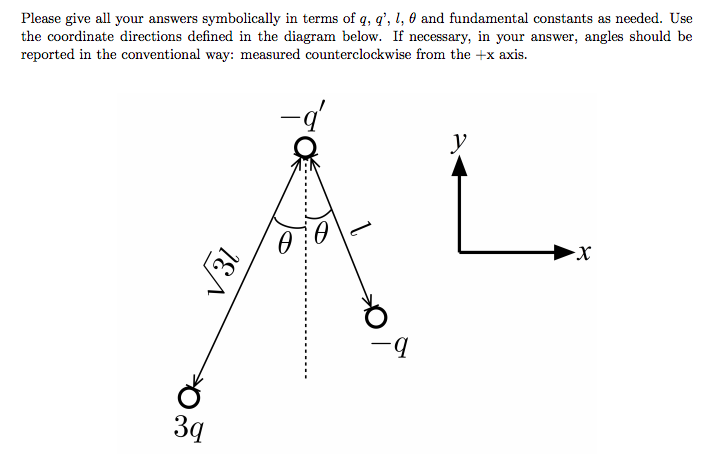
Using the graphic above, find a) the net force acting on particle -q' and b) the direction of the net force on this charge.
Step 1: Calculate the net force.
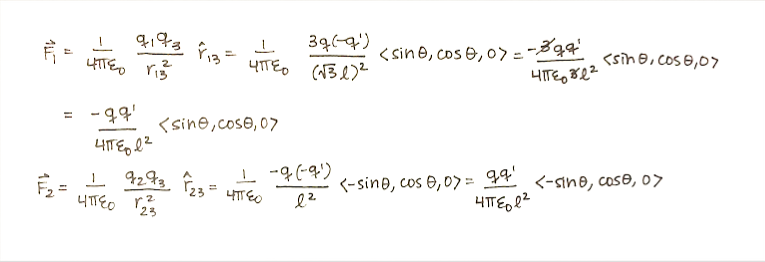
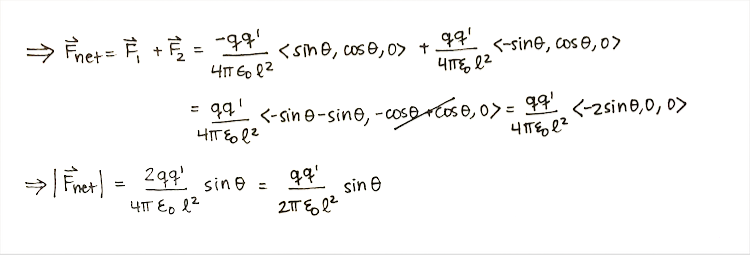
Step 2: Evaluate the direction of the force.

Problems involving electric force exclusively will not be more complicated than the above. However, the the electric force can be used in calculation of a net force acting on a particle in combination with non-Coulomb electric force and magnetic force.
Electric force is ubiquitous in everyday life, although it is not always evident. One interesting example of the electric force is the attraction of clothes to one another after being washed. The charges caused by the machine-drying process create opposite, attractive charges on different pieces of clothing which cause them to stick together. Another example can be seen in refrigerator magnets due to the electromagnetic forces that enable magnetism.
I am a physics major, and as such I must consider the electric force in a lot of different situations, and not just obvious ones. The electric and magnetic fields around ultra-dense objects such as neutron stars and black holes cause electrons to accelerate, and create radiation, for example. Knowing how to treat the electromagnetic force is extremely helpful in understanding various phenomena in Space.
Thinking more complexly, the electric force is also prevalent in almost all forms of modern technology involving electricity. One particular example is the process of charging a smartphone: the electric force allows a current to be generated which transfers charge from outlets to the internal battery of these devices. A final, slightly more complicated example of the electric force is seen in the production of abrasive paper, whereby positively charged-smoothing particles are attracted to a negatively charged, smooth surface to create papers like sandpaper.
French physicist Charles-Augustin de Coulomb discovered in 1785 that the magnitude of electric force between two charged particles is directly proportional to the product of the absolute value of the two charges and inversely proportional to the distance squared between the two particles. He experimented with a torsion balance which consisted of an insulated bar suspended in the air by a silk thread. Coulomb attached a metal ball with a known charge to one end of the insulated bar. He then brought another ball with the same charge near the first ball. This distance between the two balls was recorded. The balls repelled each other, causing the silk thread to twist. The angle of the twist was measured and by knowing how much force was required for the thread to twist through the recorded angle, Coulomb was able to calculate the force between the two balls and derive the formula for electric force.
This video explains Coulomb's experiment and the corresponding derivation of his law.
It wasn't until much later, however, that the fundamental charge (the charge on an electron) was discovered when Millikan ran his oil drop experiment in 1913 that this value was determined.
Matter & Interactions, Vol. II: Electric and Magnetic Interactions, 4th Edition